|
_SLIDE_1_
CLINICAL TRIAL
SURVIVAL ANALYSIS
OVERVIEW
_SLIDE_2_
CLINICAL TRIAL
SURVIVAL ANALYSIS
OVERVIEW
_SLIDE_3_
CONTENTS
1. Clinical Trials
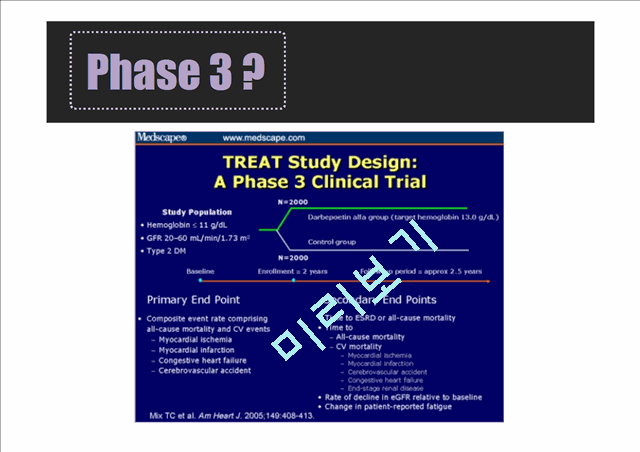
3. Phase 3
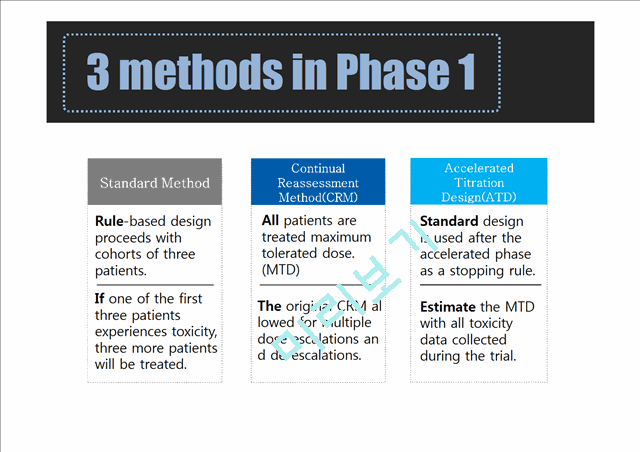
2. Phase 1
_SLIDE_4_
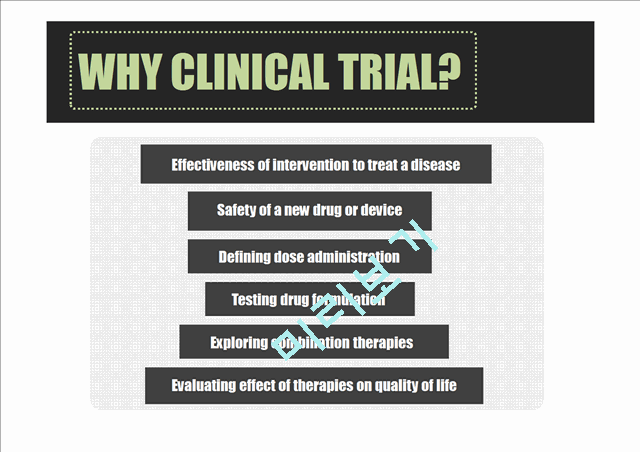
WHY CLINICAL TRIAL
Effectiveness of intervention to treat a disease
Safety of a new drug or device
Defining dose administration
Testing drug formulation
Exploring combination therapies
Evaluating effect of therapies on quality of life
_SLIDE_5_
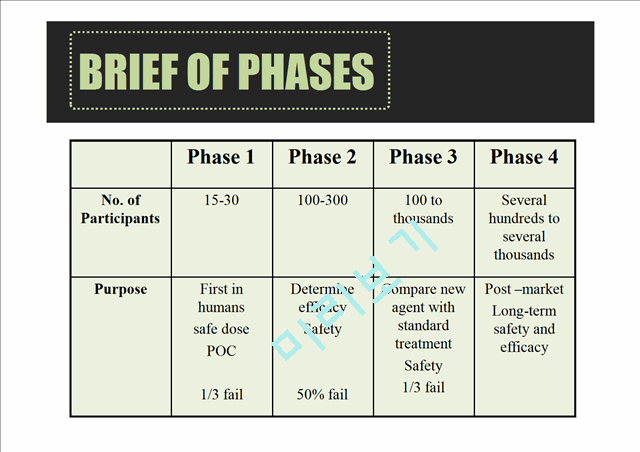
BRIEF OF PHASES
Phase 1
Phase 2
Phase 3
Phase 4
No. of Participants
15-30
100-300
100 to thousands
Several hundreds to several thousands
Purpose
First in humans
safe dose
POC
1/3 fail
Determine efficacy
Safety
50% fail
Compare new agent with standard treatment
Safety
1/3 fail
P…(생략)
|